As the cost and timeline of bringing a new drug to market continue to increase, process efficiency has become a key goal for pharmaceutical manufacturers across the industry. Innovative solutions, such as closed processing and single-use technology, developed over the last decade have helped drive better economics and accelerated scale-up. However, a critical component of successfully doing so is not just implementing these tools but also combining them with an optimal facility layout and effective resource scheduling. This holistic approach to improving operational efficiency streamlines the most fundamental elements of a business for long-term cost-saving and productivity gains.
Recognizing the benefits of such a strategy, MilliporeSigma partnered with IPS to evaluate existing cell banking processes at their Rockville, Maryland site to find innovative solutions to potential inefficiencies and bottlenecks caused by constraints such as footprint restrictions and open processing. Utilizing their expertise in commissioning, qualification, and validation (CQV), cell-based bioprocesses, architecture, engineering, and construction, IPS team members interfaced with MilliporeSigma’s operational group to design new layouts and processes that would optimize the facility’s flow and throughput.
Case Study: MilliporeSigma Facility Redesign
IPS began the optimization and scale-up effort with MilliporeSigma by conducting remote observations to study their GMP suite processes and monitor each step of how they create cell banks in a GMP environment. Specifically, IPS focused on the GMP cell banking portion of the manufacturing process, where MilliporeSigma operators relied on hands-on manufacturing operations within their cell banking suites. Several areas of the existing process were constrained by footprint and time-consuming open process operations, leading to bottlenecks that impacted overall efficiency.
The first step was media preparation, where nutrient-rich solutions are produced and sterile filtered as a raw material to promote the cell growth. The next step was cell culture, where mammalian or insect cells were thawed and expanded in conventional vessels and grown in incubators. Once the cells reached the target quantity for the cell bank, they were pooled, harvested, and formulated for cryopreservation with the aid of benchtop centrifuges. Due to the limited capacity of the benchtop centrifuge, multiple centrifugation steps were necessary to harvest the entire volume of cells required to constitute a cell bank while maintaining cell viability. After preparation of the final suspension, the cells were manipulated in a biosafety cabinet via manual pipetting and filled in vials for cryopreservation.
Figure 1, created by the IPS process engineering team using Autodesk Revit™ software, illustrates the layout of the facility for this operation. MilliporeSigma’s facility had seven nearly identical GMP banking suites utilizing a unidirectional flow design.
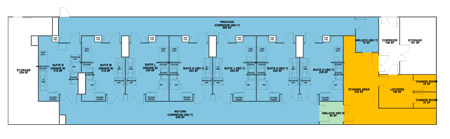
Figure 1: Current-State Layout
Four of the suites were ISO Class 7 cleanrooms, and the remaining three were EU Grade B. Each suite was designed for and included the necessary equipment to process an entire batch within a single suite.
IPS Proposal A: Optimized Cell Banking Process
The initial proposal designed by the IPS process engineering team was an optimized version of MilliporeSigma’s existing process. The proposal included a closed format with single-use materials that was intended to de-risk operations and enable processing of multiple batches in the same space, thereby improving output. It transitioned to a closed process at the earliest possible point, immediately following cell thawing/inoculation. This was done in a closed vessel within an ISO5/Grade A biosafety cabinet in an ISO Class 7/Grade B room background, which is appropriately classified for open work in a biosafety cabinet.
From there, the process moved to a Grade D ballroom suite, where manipulations would occur on an open bench within a series of closed systems. Closed processing utilizing single-use tubing sets, sterile tube welders, and sterile tube sealers to make aseptic connections makes it possible to operate in a lower room classification while still sufficiently protecting the product. In the first step in the ballroom suite, the cells would undergo a series of expansion steps in closed shake flasks until the target cell count was met. Then, the MilliporeSigma team would continue to grow the cells to higher quantities in a rocker bioreactor system, facilitating production of larger volumes. Next, a continuous single-use centrifuge would be used to harvest and wash the cells before formulation in cryopreservation agents. The harvested material would then be passed to a dedicated filling suite housing an automated system to fill and seal individual vials for cryopreservation. Figure 2 (also created using Autodesk Revit™) shows the revised floor model for the proposed process.
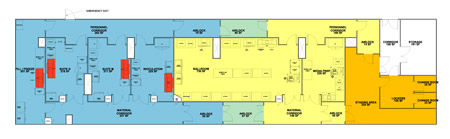
Figure 2: Proposed Layout A
Rather than the previous setup of seven parallel suites, the facility would now include a media preparation suite dedicated to the cell banking operation, complete with mixers and appropriate storage space; an inoculation suite, dedicated to thawing and closing the process for each batch; a ballroom suite with multiple bioreactors for parallel processing of different batches; a filling suite with automated vial filling and sealing equipment; and two flex suites for either legacy cell banking operations or additional inoculation capacity for the ballroom process. The inoculation suite in Figure 2 is where the thawed material is passed into the declassified ballroom. The ballroom uses the space that previously contained three suites (one product per suite) and replaces it with one that can house up to ten parallel banks for simultaneous processing.
IPS Proposal B: Optimized Cell Banking Process with Limited Construction
IPS’ second concept for the revised cell banking process was a value-engineered option for MilliporeSigma that reduced the cost and construction required for the initial proposal. Figure 3 illustrates this second option.
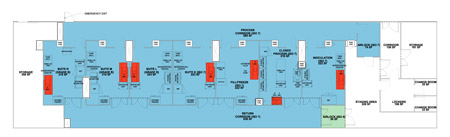
Figure 3: Proposed Layout B
In this setup, the inoculation suite is now on the far right, where the cells are thawed, prepared, and then passed through a closed processing suite that houses up to six incubators. Manipulation takes place on an open bench, and the cells are then harvested in the same space before being moved to the filling suite. Although it does not achieve the same output and total banking capacity as the initial proposal, this option does use the same principles of a closed process.
With the layout and material flow diagrams, the IPS industrial engineering team used commercially available, discrete event simulation software (AnyLogic) to compare personnel and equipment utilization across all three options (the current state of operation versus the revised layouts designed by the IPS process engineering team). First, they identified what resources were in use at each step of the current process and then coded the process flow into AnyLogic, including a visualization of the actual workflow (Figure 4).
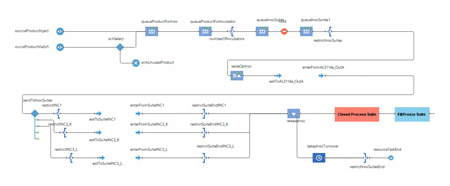
Figure 4: Simulation Code Example
MilliporeSigma provided IPS with the real-world throughput achieved over the preceding year, enabling the IPS industrial engineering team to compare the output of the model to the real-world data. Coding adjustments were then made to the current-state model to achieve accurate approximations of the throughput. It is important to note that the AnyLogic model created evaluates the “best-case" scenario and did not consider some potential outliers, such as a change in personnel or issues with raw materials that create unexpected delays. While these outliers can be simulated using AnyLogic, it was not necessary when comparing equivalent scenarios across all three models.
With an accurate current-state model as a foundation, new models were generated to reflect the proposed ballroom (Proposal A) and value-engineered (Proposal B) layouts. The updated models took the parallel operations that were occurring simultaneously across different suites into account. For example, because the ballroom suite is designed to run so many parallel operations, it was unlikely that the single inoculation suite would be able to supply enough thawed cell cultures to maintain a high utilization of equipment within the ballroom, especially when taking changeover and cleaning into consideration. IPS adjusted the program to provide data for scenarios when only one inoculation suite was being utilized versus when two and then three suites were used to thaw and inoculate cells.
IPS also used AnyLogic to change the range of incubation times and the number of incubation passages. This functionality is different than other programs, such as Excel. Excel may allow for the same capture of data, but it does not easily allow for randomness and it does not account for potential changes in data based on adjustments to previous steps. These factors are some of the important aspects that allow discrete event simulation software like AnyLogic to accurately simulate unpredictable events. Table 1 shows the final numbers based on output from the various options (i.e., the current-state, the ballroom, and the value-engineered) under different simulations, varying the number of closed inoculation suites versus open processing suites.
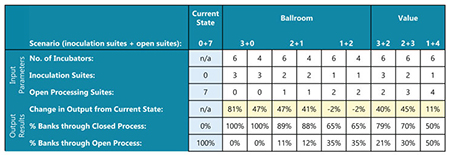
Table 1: Results of Simulation Scenarios
Initially, two dual-stack incubators were designed in the first step of the ballroom process (for a total of four incubators), but after evaluating the simulation, IPS and MilliporeSigma recognized this as a bottleneck. Therefore, they increased the number of dual-stack incubators to three (for a total of six incubators), which allowed for six parallel cell culture operations to occur at the same time. In this state, with three inoculation suites being utilized, there was an 81% increase in the number of cell banks completed in one year (when compared to the current-state model throughput).
With four incubators, the increase is 47%. If the setup has only two inoculation suites and one for legacy open processing with six incubators, there is still an increase of 47% with six incubators (41% with four incubators). However, there is no throughput increase when there is only one inoculation suite. In the value-engineered option, two inoculation suites and six incubators allow for a 45% increase, which is roughly the same as the ballroom option, with a majority of the investment in equipment rather than architecture and renovation. While one of the goals of the study was to optimize operator scheduling, additional efforts to do so (i.e., scheduling software) were no longer necessary, as the efficiency of the ballroom option ultimately increased the output per operator by 10%.
The increased efficiency and facility output modeled in this case study exemplify how collaborative interactions across multiple IPS disciplines and client stakeholders yield measurable improvements. Moving forward, IPS continues to support MilliporeSigma by assisting as necessary with the implementation of the new workflow. This includes additional consulting and process development support, such as revising batch record templates and standard operating procedures, designing and sourcing components and consumables, procuring equipment, and executing the facility renovation.
Discrete Event Simulation Modeling in Pharmaceutical Operations
Advancements in science and technology continue to revolutionize patient care, bringing new and innovative products to the market every day. However, finding ways to overcome the complex development and manufacturing challenges these therapies present is critical to ensuring the delivery of safe and effective treatments. This includes facility, workflow, and resource optimization, as enhancing operational efficiency allows drug companies to adapt their production life cycles to the closed processing and automated systems that are more prevalent in today’s changing landscape while also achieving lower costs and faster speed to market.
Traditionally, internal expertise at a pharmaceutical company can be used to identify process intensification solutions that drive higher quality drugs to be produced in a more cost-effective way. Yet, the collaboration between IPS’ process and industrial engineering experts, combining bioprocess expertise with discrete event simulation, offers an innovative option that helps quantify and predict the optimal functionality of a facility and what resources would be necessary to achieve that output. By integrating the disciplines of the process development and industrial engineering teams ― which include a wide range of expertise in biological processes, functionally closed automated systems, manufacturing, aseptic filling technology, and more ― IPS experts are able to gather the insight necessary to deliver significant improvements critical to the success of its biopharma clients. This, combined with improved regulatory compliance achieved by leveraging IPS’ CQV disciplines early in the design effort, helps anticipate regulatory hurdles and offers a major advantage to companies vying for a place in an increasingly competitive market.
Read the article on CellandGene.com.



