The demand for cell and ex-vivo gene therapies is steadily increasing, given the impressive efficacy of autologous chimeric antigen receptor T-cell (CAR-T) therapies against hematological malignancies. In recent years, the reality of curative treatment and commercial approval of several autologous CAR-T products triggered developers to plan for increased capacity and treating larger patient populations. Pioneers in the CAR-T therapy space are also heavily investing in allogeneic platforms to create off-the-shelf therapeutic cells that are immediately available and can treat a large number of patients with a single product batch.
This is the story of one such pioneer and their journey to enable late-stage clinical and commercial programs. Although our client’s company name will be kept confidential, this article will disclose the approach to finding their home for end-to-end allogeneic CAR-T therapy manufacturing.
Drivers for Commercial Expansion
How does a cell therapy drug developer decide to commit to the major undertaking of building a commercial facility? This is a costly and time-consuming endeavor, so the reward must be worth the risks. The chart below shows the landscape of preclinical, clinical, and commercial cell therapy programs in 2021 and 2022; we can appreciate that the industry is growing rapidly, and demand for GMP manufacturing space is at an all-time high.
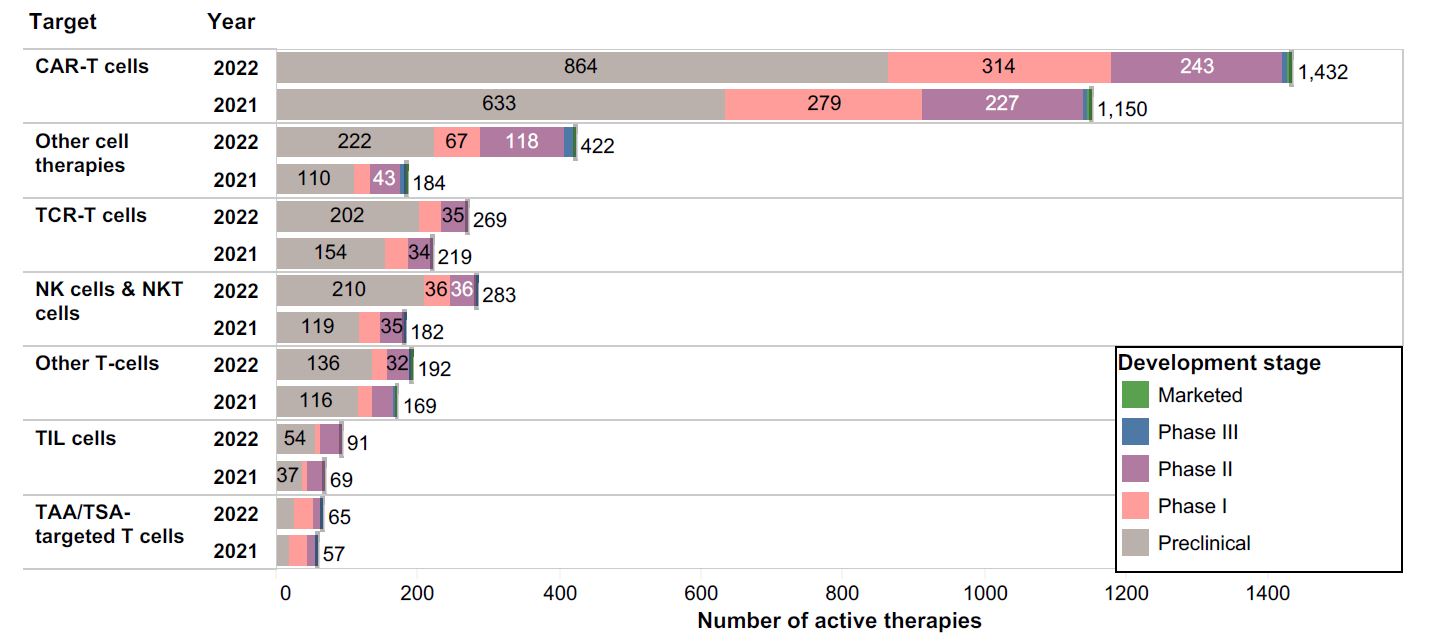
Figure 1. Source: https://www.cancerresearch.org/cancer-cell-therapy-landscape
Snapshot of active immune-oncology cell therapy programs as reported by the Cancer Research Institute, including preclinical, Phase I – III Clinical, and Marketed products.
Realizing success in the clinic, desire for in-house manufacturing, future growth targets, and a growing list of required supporting functions are strong motivators for drug developers to invest in their manufacturing capacity as a product advances towards late-stage development.
Early clinical efficacy breathes life into a program and often helps secure significant capital investment to fund the design and construction of a GMP facility. Higher manufacturing capacity becomes necessary to enable Phase II/III clinical trials; hence preparedness for expansion should be considered soon after positive Phase I results. The table below paints a picture of a typical facility architecture program progressing from Phase I to commercial manufacturing:
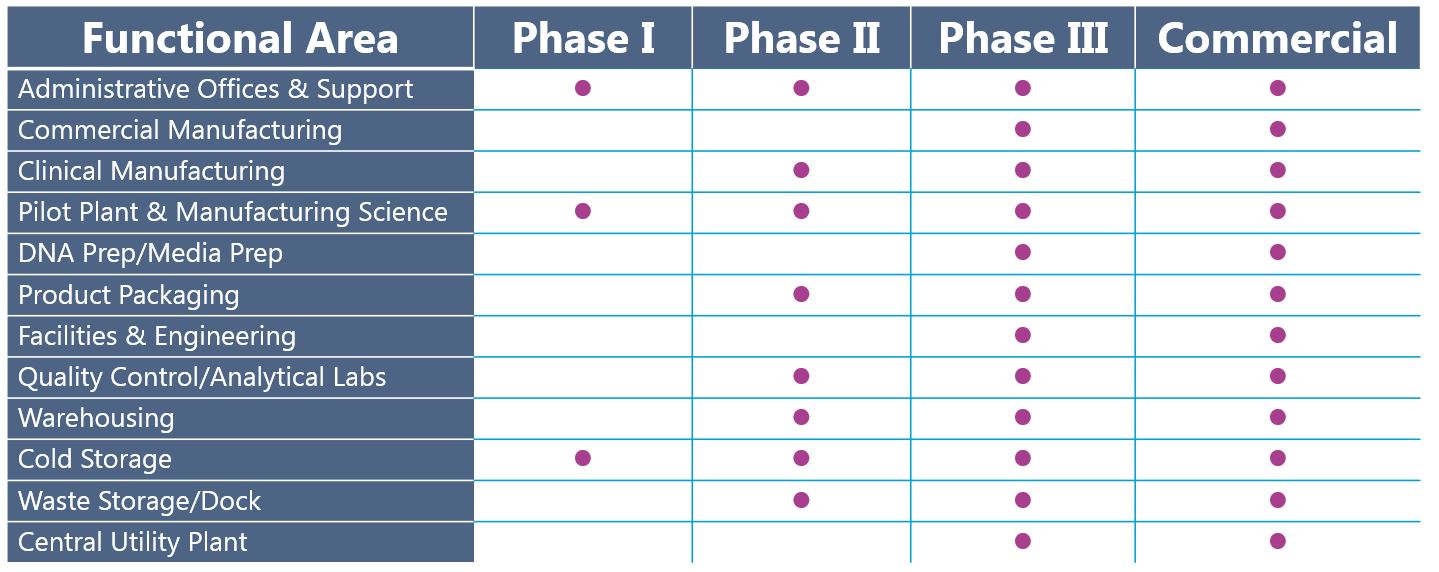
Figure 2. Snapshot of facility functional areas required to support Phase I, Phase II, Phase II, and Commercial manufacturing.
Facilities producing Phase I materials are relatively simple. Supporting functions such as quality control, additional warehousing, waste storage, and a pilot plant are necessary to house onsite in Phase II and beyond. By Phase III, the facility producing your drug product closely aligns with the needs of a commercial plant. Planning for commercial manufacturing capacity concurrently with Phase I or sooner enables developers to design, construct, and commission manufacturing space without lengthy delays to the late-stage clinical program.
Is it better to manufacture your product in-house or at a CDMO site? For companies wishing to control the manufacturing process closely, maintaining the operation in-house assuages intellectual property concerns and allows for quick responses to issues as they arise. Furthermore, cell therapy manufacturing space at a CDMO can be tough to secure, often requiring booking slots years in advance.
In this case study, the client aims to deliver their allogeneic product to thousands of patients with plans to increase patient populations in Phase II and III clinical trials. In addition, future products destined for the clinical pipeline will only increase demand for manufacturing capacity. For this and the above reasons, our client designed and built their manufacturing facility.
Informing the Business Case
New projects require funding and a business plan to obtain it. A significant part of any business plan for a manufacturing facility is understanding the expected cost and timing for commercial manufacturing. It all starts with the product and the definition of the required capacity. With these goals in place, a high-level study can be performed ahead of conceptual design to define the process and establish the accurate requirements for a fit-for-purpose facility.
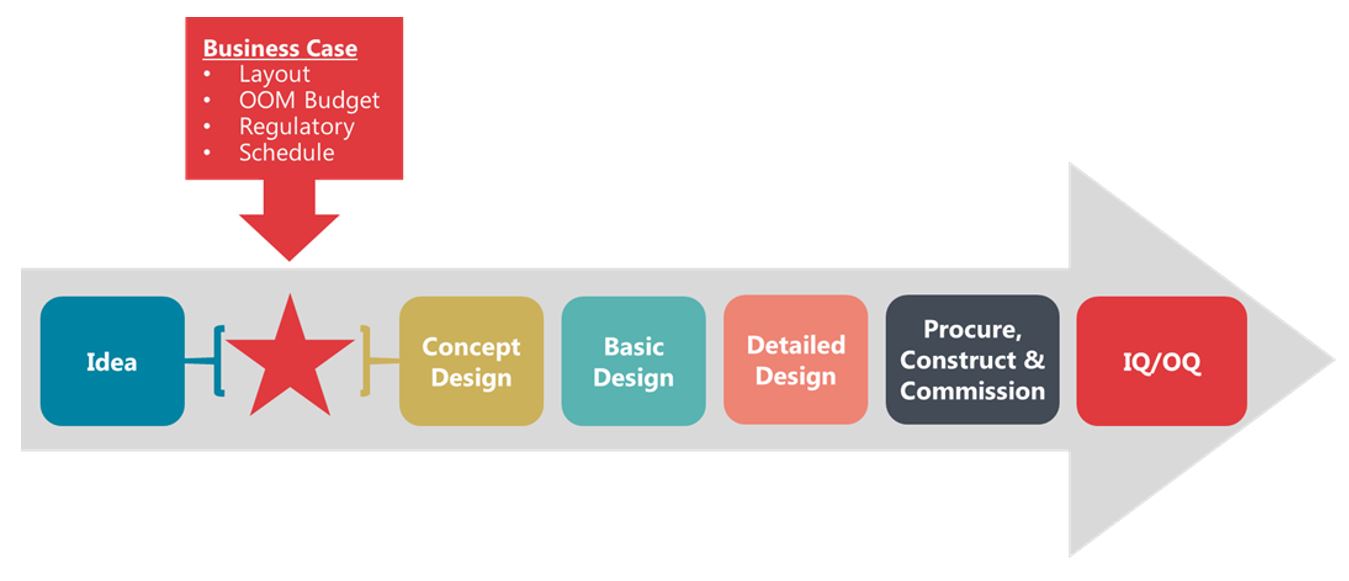
Figure 3. Diagram illustrating the flow of business case development and subsequent facility design, construction, and qualification process.
Timing is often critical with any business case justification as the windows for project approvals can be few and far between. In lieu of lengthy engineering exercises required to produce design packages, teams must rely on a collaborative approach with experienced design partners to quickly define the manufacturing process and establish the facility requirements. In this case, the client sought to purchase a facility, so the premise was based on defining what was needed to support their real estate search.
Design Process
For an efficient study, a hierarchy of basic project principles needs to be established to bring focus to the design process and alignment of the project team with the stakeholders. These principle components must be ordered based on their ability to affect subsequent areas of the design and, ultimately, the project’s outcome. For life science facilities, these essential components are Product, Process, and Facility. As mentioned above, the definition of the product or product types is primary. The products ultimately dictate the manufacturing process, establishing the facility’s requirements.
Product
The product is profiled as ex-vivo genetically modified allogeneic immune cells engineered to target various hematologic malignancies and solid tumors. Allogeneic therapeutics enable the treatment of a large patient population with a single batch by first creating a bank of immune cells isolated from the donor and using that cell bank to create multiple off-the-shelf batches of the same product. An externally sourced biosafety level 1 (BSL-1) viral vector transduces and engineers the cells. The final product will be filled into vials with a target output of 500-600 vials per batch.
Process
After receiving starting material, the client’s manufacturing process first isolates immune cells. It expands those cells to create a master cell bank (MCB), the common stock of starting material used to initiate multiple allogeneic product batches. The workflow depicted below involves closed cell expansion of the isolated immune cells ranging from shake flasks to bioreactors, closed harvest utilizing continuous flow centrifuge, and automated vial fill inside an isolator. The MCB is then cryopreserved in a controlled-rate freezer (CRF) and stored in liquid nitrogen (LN2) freezer.
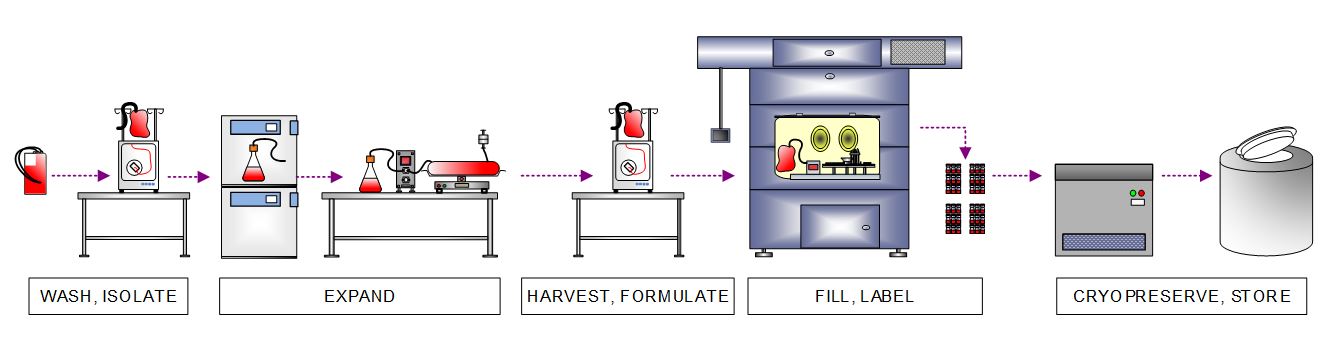
Figure 4 . High-level process flow diagram depicting immune cell isolation, expansion, harvest, formulation, filling, and cryopreservation to create a MCB that will be used to produce multiple allogeneic batches.
Batch production is initiated by first thawing MCB vials and engineering the cells with a recombinant viral vector delivering the proprietary CAR gene. All engineering steps are performed with closed, single-use components, including closed culture vessels, CO2 incubators, rocking platforms, tube sealers/welders, and peristaltic pumps.
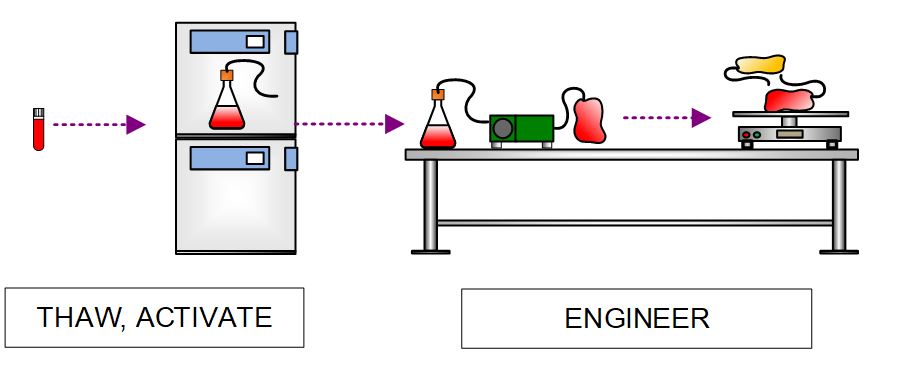
Figure 5. High-level process flow diagram depicting thaw and activation of MCB and manipulations to introduce CAR gene with a viral vector.
Engineered cells are then expanded in multiple bioreactors. Supporting equipment includes a cell counter, bio-analyzer, heat exchanger, and chiller. Once the desired product cell density is reached, the reactor harvests are pooled in a bioprocess container. Cell wash and product formulation is performed in a continuous flow centrifuge.
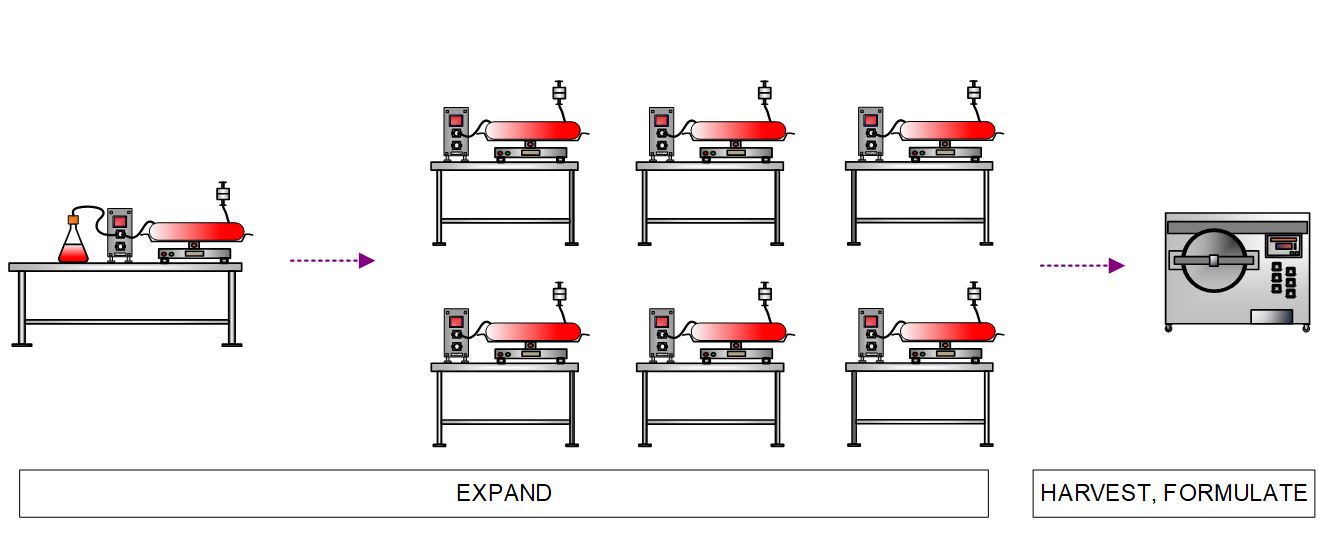
Figure 6. High-level process flow diagram depicting expansion, harvest, and formulation of engineered allogeneic CAR cells.
Once cryopreservative is added to the cells, there is a narrow window of 1-2 hours during which the product must be filled, inspected, and labeled so the cryopreservation cycle can be started in time to avoid damage to the cells. Our client’s batch size required an automated fill line capable of processing 250-1,000 vials per hour to allow enough time for labeling and cryopreservation. The fill line was housed in an isolator, and similar to creating the MCB, final product vials are cryopreserved in a CRF and stored in an LN2 freezer.
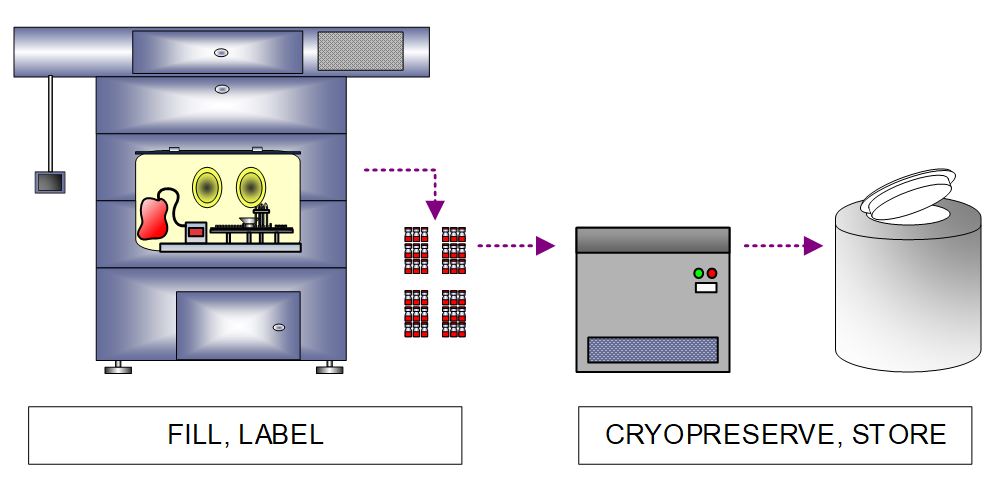
Figure 7. High-level process flow diagram depicting allogeneic CAR cell product filling, labeling, and cryopreservation.
Facility
As the process was being defined, the architects started to develop the facility program and discussed process architectural design philosophies. The facility program defines all the rooms and spaces that will be required, while the design philosophies establish guidelines for adjacencies, vertical integration, and GMP (personnel and material flows. Armed with an understanding of the process, a facility program, and design philosophies, the architects developed site-agnostic preliminary layouts of the manufacturing core as well as the surrounding spaces, which in turn defined the parameters for the building search.
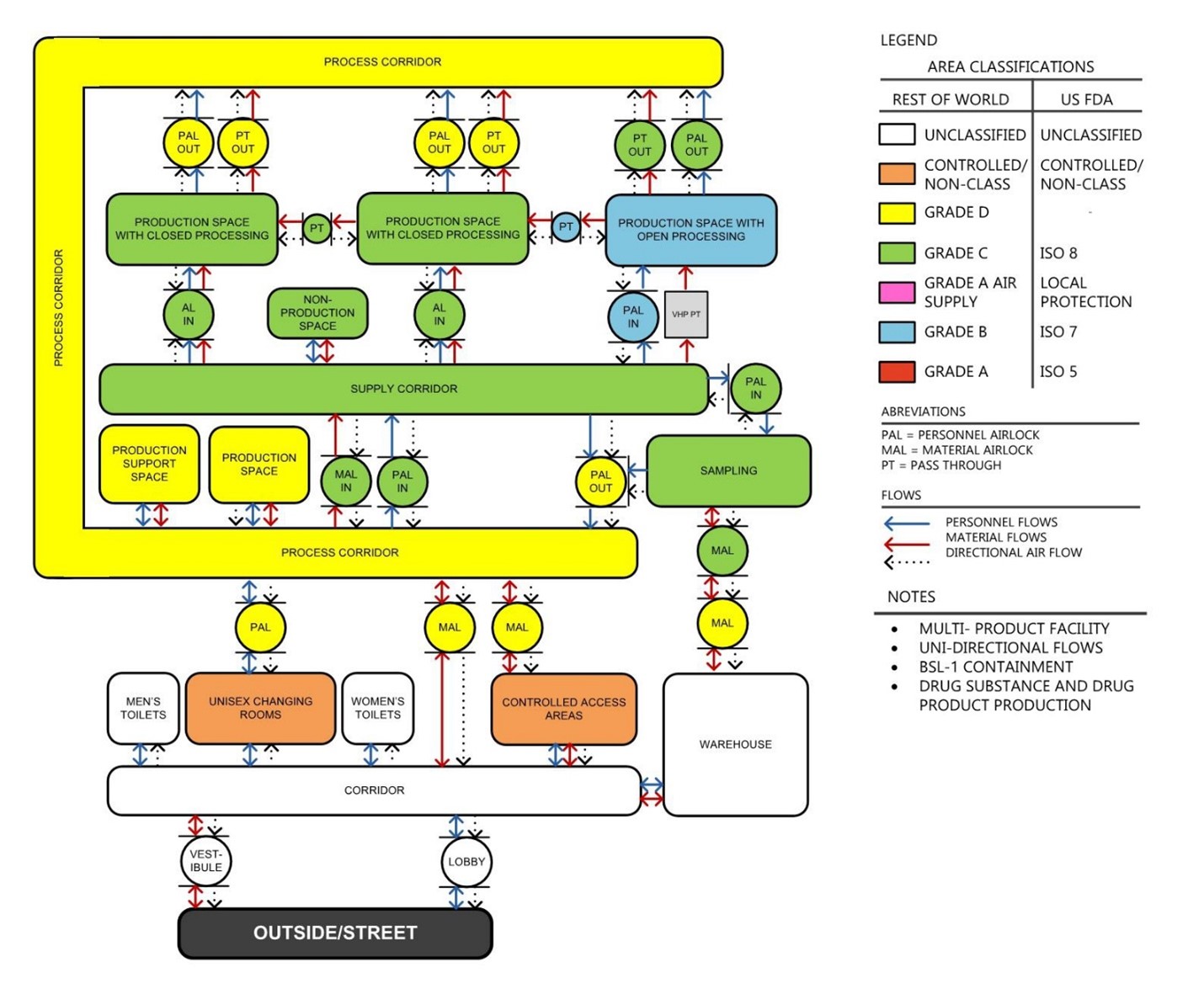
Figure 8. Zoning & Transition Diagram defines GMP areas classifications, operational flows, and directional airflow. It establishes the rules that the layout should follow.
At this point, the team had all the information they needed to assess the total costs for the project, which were broken down into expected renovation costs, equipment costs, various site work allowances, and owner costs. A milestone schedule was also created to define the expected project timeline.
The final output of the study was then folded into the business plan. It summarized the products, the expected capacity, facility requirements, project costs, and overall timing. The business plan was approved, and it was time to find a home.
Site and Building Selection
A number of factors must be considered when choosing a building to renovate for life sciences. Municipal zoning must be designated for manufacturing applications at the most primary. In addition, the building should be designed to support factory industrial practices, as traditional office buildings are not usually capable of supporting the movement of heavy equipment or the vibration control for sensitive equipment.
Structural spans and floor-to-floor heights also play a crucial role in maintenance accessibility and the development of optimal layouts. It is common to use prefabricated, modular cleanrooms because they suitably maintain a clean environment. They often have the added benefit of a walkable ceiling, which is hung from the floor or roof above and can be used for maintenance activities. If the building steel is not strong enough to support these walkable ceilings and if the vertical height is not tall enough, the benefits of these modern systems cannot be fully utilized, and a traditional stick-built approach becomes a more attractive option. A dense building with columns spaced closely together will also constrict optimal room layouts, adjacencies, and equipment arrangements.

Figure 9. Cleanroom construction approach options.
Option 1: modular with supporting utilities mounted on cleanroom ceiling. Option 2: modular with supporting utilities hung from the building structure above. Option 3: POD-based cleanroom construction with utilities hung from the building structure above. Option 4: traditional stick-built facility.
Another determining factor is the size of the lot and access roadways. There must be enough parking for the estimated occupancy and the possibilities for truck access and deliveries. Space should also be assessed for outside utilities, such as emergency generators or mechanical systems.
Location is crucial. Costs can vary widely from one place to another. This can affect the cost of real estate as well as construction costs. The location must also consider the workforce and if there is a talent pool of skilled labor to draw from.
The top building choice was within an existing science park. Although the location was perfect for obtaining talent, the building did lack the optimal floor-to-floor heights and was slightly undersized to accommodate the entire program. The design team performed test fits to see how much of the total program could fit in the facility, and decisions were made to reduce the program. The building was ultimately purchased, and the design team adapted the facility to produce allogeneic CAR therapies.
Final Design Effort
Leveraging the work performed in the tested fits, a complete conceptual design was done to define the process further and create layouts for the three-story building. Manufacturing was located on the ground floor because it was best suited for material movement and vibration control. The offices and laboratories were located on the upper floors. The project progressed through detail design and is now nearing construction completion.
Lessons Learned
When working with novel product modalities, the team must remain flexible to adapt to the ever-evolving science and product development. Process changes should be implemented as soon as possible to minimize rework and the impact on downstream systems. Site and building selection are crucial. The building must be fit for purpose and capable of adapting to the requirements of a manufacturing building. In this example, the existing building was designed to support labs and office space, which made it challenging to incorporate the complex mechanical and utility systems of a GMP facility. Owners should invest in due diligence activities to adequately assess potential properties, as developers are not always aware of the requirements for these highly regulated facilities.
Conclusion
The development of cell and gene therapies are on the rise due to their potential to create effective treatment options for lethal malignancies and disorders. As these products move from clinical development to commercial manufacturing, owners must carefully consider the GMP requirements for producing their products and the facilities that house them.



