Many countries, including the U.S., lack the necessary surge capacity to reliably fill-finish and deliver billions of incremental doses of injectable drugs and vaccines during bio-emergencies.
Most injectable drugs are administered with disposable syringes, filled from single- or multi-dose glass vials that are filled and finished in bulk by complex, international supply chains. Unfortunately, these fill-finish operations typically lack the capacity for rapidly scaling up or down in emergencies. Additional filling lines cannot be built quickly. Even if they could, investing substantial capital in expansion during emergencies may be unwise if it leaves excess capacity after demand reverts to pre-emergency levels.
ApiJect identified Blow-Fill-Seal (BFS) technology as having more favorable features for surge manufacturing than traditional glass filling lines (high output, fast scaling, cost-effective). And by attaching a pen needle-style hub to the top of the BFS Container, the assembled drug delivery system (i.e., the ApiJect Prefilled Injector) creates a new type of scalable and cost-effective prefilled syringe. We believe that in the future, BFS drug delivery systems like the ApiJect Prefilled Injector will become an increasingly popular format for injectable drugs, growing the overall prefilled syringe market and making these large capital investments a smart business decision.
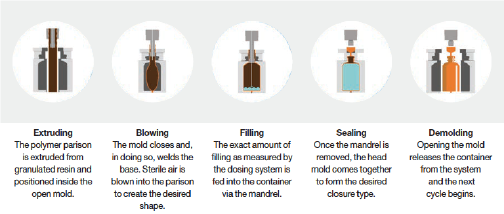
BFS presents a cost-effective option for single-dose delivery of vaccines. BFS has evolved over 60 years to become a trusted and relied upon way to package sterile liquid pharmaceuticals. Its versatility enables companies to package dose volumes ranging from 0.2mL to more than 500mL in polymer containers of a wide range of shapes. The efficiency of BFS allows for both large and small orders to be fulfilled with reliable quality at high speeds and volumes. The aseptic BFS filling process enables a single machine to perform a continuous sequence of automated operations to mold plastic containers out of molten resin (LDPE in ApiJect’s case), fill the containers with sterile liquid, then cool and seal them in under three seconds.
 Benefits
Benefits
- Product Integrity
- Flexible Container Design
- High Product Output
- Low Operational Costs
- Enhanced Safety
- Quality Fill-Finish
- Low Overfill Rate
These steps are performed in a highly controlled environment, generally regarded as an ISO-5 area, with no human intervention during the aseptic filling process, helping to maintain product sterility. And BFS is highly scalable; each Rommelag bp460 machine ApiJect uses can fill and finish up to 15 million finished doses per month. BFS is used to annually package tens of billions of units of eye drops, oral vaccines, biologics, and other sterile liquid pharmaceuticals… but until now, not in a ready-to-use, mass market, prefilled syringe format.
ApiJect’s technology enables BFS to potentially become a real factor in the prefilled syringe market with the ApiJect Prefilled Injector.
Overview: Project Jumpstart
Project Jumpstart is a short-term emergency program, managed by ApiJect and created by the U.S. Department of Health and Human Services (HHS) and the Department of Defense (DOD), to establish temporary fill-finish capacity for up to 45M doses a month of COVID-19 vaccine on BFS lines by the end of 2020. All of these BFS-packaged doses could then be used in ApiJect Prefilled Injectors for scalable, singledose delivery. To achieve this, ApiJect agreed to upgrade three existing BFS lines in a U.S. contract manufacturer (previously making single-use eye drops or other non-injectable products) to run ApiJect BFS molds and fill-finish under BSL-2 conditions. ApiJect met the HHS/DOD timetable for Project Jumpstart. The first BFS line became operational on October 30, 2020, less than seven months since the project launch. The second BFS line became operational a month later and pharmaceutical partners began engaging with ApiJect about using this fill-finish capacity.
Under Project Jumpstart, ApiJect and the U.S. now have domestic surge capacity to fill-finish 45M BFS Containers per month with sterile liquid pharmaceuticals within a BSL-2 environment.
As of Q1 2021, the Needle Hub and Connector components of ApiJect’s Prefilled Injector have been submitted for FDA review with the intention to be paired with a COVID-19 vaccine in the BFS Containers produced on the Project Jumpstart lines.



