The ApiJect Prefilled Injector, a scalable, cost-effective type of prefilled syringe, is made possible by the ApiJect Technology Platform, which unlocks the potential of blow-fill-seal (BFS) packaging to solve a wide range of drug delivery problems. The platform’s interconnecting-module design parameters, together with its pre-existing component base, is configured to help pharma companies design and manufacture drug delivery systems that can be scalable, safe, and cost-effective for markets worldwide.
 The ApiJect Technology Platform Expands BFS Possibilities
The ApiJect Technology Platform Expands BFS Possibilities
At its core, the ApiJect Technology Platform intends to support the design and manufacture of simple, primarily plastic components that attach to ApiJect-approved BFS containers. This is designed to enable the creation of versatile drug delivery systems for liquid pharmaceuticals that can be assembled automatically at the manufacturing facility or manually in the field by the healthcare professional immediately prior to drug injection.
By embracing this design philosophy, pharma companies can rely on an ApiJect-approved BFS line either in their facility or a trusted third party to aseptically fill and finish their sterile liquid drugs into a drug delivery system made on the platform – creating a very flexible manufacturing approach to help address their drug delivery challenges.
Introducing the ApiJect Prefilled Injector
The first device created on the platform, the ApiJect Prefilled Injector, illustrates the platform’s potential for how BFS can impact the prefilled syringe market.
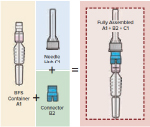
 The prefilled injector brings together a 0.5mL dose-filled BFS container with a plastic pen needle-style hub to create a new type of prefilled syringe. By employing two scalable, cost-effective technologies – BFS (for the drug container) and injection molding (for the attachable components) – the ApiJect Prefilled Injector has the potential to enable injectable drugs that typically would not be considered for a prefilled syringe format due to prohibitive economics to now be filled and finished in a single-dose drug delivery system with speed, scale, and economic efficiency.
The prefilled injector brings together a 0.5mL dose-filled BFS container with a plastic pen needle-style hub to create a new type of prefilled syringe. By employing two scalable, cost-effective technologies – BFS (for the drug container) and injection molding (for the attachable components) – the ApiJect Prefilled Injector has the potential to enable injectable drugs that typically would not be considered for a prefilled syringe format due to prohibitive economics to now be filled and finished in a single-dose drug delivery system with speed, scale, and economic efficiency.
This is one example of how the ApiJect Technology Platform is designed to help pharma companies to deliver more sterile liquid pharmaceuticals to more patients in more markets.
Potential Benefits of ApiJect’s Technology
Potential benefits of the ApiJect Prefilled Injector technology include:
- Supply Chain – The ApiJect Prefilled Injector utilizes a compact, reliable supply chain that only uses two primary ingredients: pharmaceutical-grade resin and hypodermic needles.
- Scalable – A single BFS line can potentially fill and finish up to 15 million units a month with almost no human intervention.
- Aseptic Quality – ApiJect currently utilizes rotary BFS machines that aseptically fill and seal the drug dose in what is generally regarded as an ISO-5 area.
- Flexible Volumes – The BFS machines used by ApiJect can fulfill both very large and much more modest order sizes. And, they can potentially switch from one drug to another in less than a day if needed.
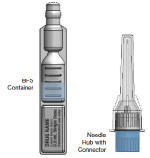
- Cold-Chain Efficiency – Since the BFS container and needle hub with connector are made separately from one another (see figure 2), only the BFS container needs to be put in the cold chain, saving space.
- Transportability – The ApiJect Prefilled Injector is lightweight, potentially saving on shipping costs. And, since it is made out of flexible plastic, it is very difficult to shatter.
- Convenience – The ApiJect Prefilled Injector is designed to come with the same convenience as prefilled syringes, potentially saving preparation and administration time while reducing dosing errors.



