
Now, under one roof, cell and gene therapy companies are able to get everything they need to quickly create a CGMP compliant facility.
Facility design, traditional or modular construction, it's all included in CarTon.
The Need for CarTon

Where speed to market is critical for saving lives, our solution simplifies the complex maze of activities required to bring a new cell and gene therapy facility to a fully operational state. CarTon contains the entire process life cycle including facility and process requirements, standardized protocol templates, QMS/SOP development, training, and other necessities for CGMP manufacturing in a global market.
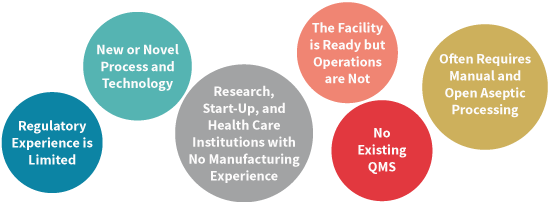
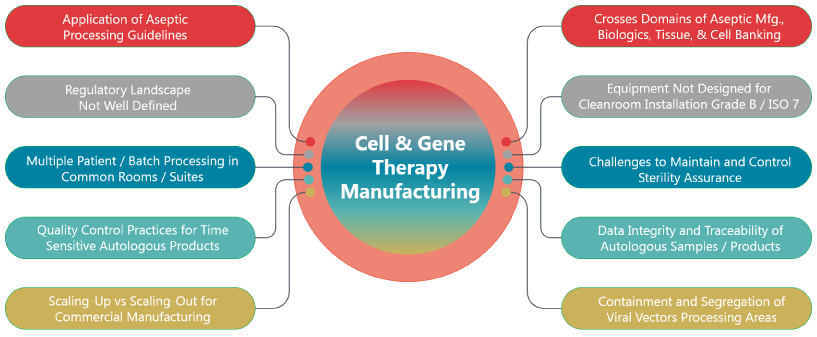
Industry and Regulatory Challenges
The CarTon includes quality by design CGMP facility and compliance; commissioning, qualification & validation; and operational readiness/quality management systems and services.
Commissioning, Qualification & Validation |
|
|---|---|
 |
|
Quality by Design CGMP Facility and Compliance |
|
|---|---|
 |
|
Manufacturing Readiness & Quality Management Systems |
|
|---|---|
 |
|
IPS Provides a Complete Turnkey Solution - Vial to Vial
With the combination of IPS’ core services, iCON, and CarTon, cell and gene therapy companies have a complete turnkey solution to get their medical brilliance to awaiting patients, iCON, the IPS and G-CON collaboration, provides for the most flexible and rapidly deployable facility platform, meeting the modular and mobile demands of the industry. The broad range of technical capabilities along with prefabricated cleanroom PODs provides a true turnkey solution to biopharmaceutical manufacturers and CMO companies with needs for production agility and capacity flexing. CarTon takes the iCON solution one-step further to a fully operational, integrated facility or as an independent operational readiness solution on any project.
|
|
|
|
| An industry leader in technical consulting, engineering, procurement, construction management, CQV, & CGMP compliance for the ATMP industry. | A turnkey facility solution for cell & gene therapy manufacturing. | A complete operational readiness solution complete in one box – The CarTon. |





